Coatings experts often say that surface preparation of a steel surface is the most important part of a coating system. By this they mean that surface preparation affects the performance of the coating more than any other variable. Given that the proper coating system has been selected, if the surface preparation is poor, coating performance will often be compromised, even when the application is perfect. If surface preparation is good, then the coating applied over it is likely to perform well. For you, the applicator, it is useful to know why surface preparation is so important, because knowing why can help you do a better job. The following discussion focuses on the preparation of steel, but the concepts apply to other substrates as well.
Surface Preparation Is a Foundation
First, we can express the reason for the importance of surface preparation in a broad, general way, with the help of an analogy or comparison. Surface preparation is to a coating system what a foundation is to a building. If a building has a poor foundation, it can list or lean, as the famous Leaning Tower of Pisa does, or it can collapse altogether. If a coating system has a poor foundation (surface preparation), it will fail sooner than expected (say, after five years rather than ten years); or it can fail cat-astrophically, within the first year of application. In both instances, reduced service life and catastrophic failure can result in great financial losses to a facility owner. The contractor may be held responsible for these losses if the surface preparation work is found to be faulty. As a professional painter, you have a responsibility to your employer to make certain that the surface preparation work you provide complies with the specification requirements—to provide the solid foundation necessary for the proper performance of the coating system. When speaking about the function of surface preparation, it is important to go beyond the general concept of a foundation, and look to specific attributes. Surface preparation creates a foundation in two important ways: a mechanical way by providing an anchor for the coating; and a chemical way by allowing intimate contact of coating molecules with the steel surface. These elements of foundation are best understood by their opposites—the negative or detrimental conditions of slipperiness and debris on the surface.
Overcoming the Negative of Slipperiness
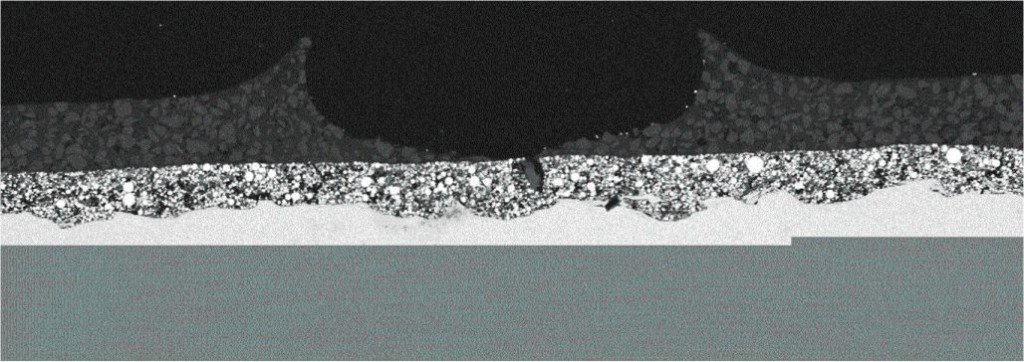
When a surface is very smooth, coatings have a difficult time adhering strongly. Imagine a coating on glass, for instance, and the ease with which it can be removed by a scraper or even a fingernail. Imagine, on the other hand, a rough surface like sandpaper on the same piece of glass, and how difficult it would be to remove a coating film from it. Steel, when it is abrasive blasted, has a surface that is rough like sandpaper, with a series of tiny peaks and valleys called surface profile (Fig. 1).
Coatings anchor themselves to the valleys of the profile, and the peaks are like teeth. This is why surface profile created by blasting is sometimes called an “anchor pattern” or “mechanical tooth.”
Overcoming the Negative of Debris
Debris on a steel surface can be comprised of many different materials. They include dirt, dust, grease, oil, rust, moisture, and in some cases, millscale. When materials such as these are painted over, they interfere with both mechanical and chemical adhesion of the coating to the substrate and make it likely that the coating will fail prematurely. On the other hand, when all debris is removed, the coating can achieve complete and continuous contact with the steel substrate, thus assuring the best possible adhesion. When a coating adheres well, it will create a more effective barrier, minimizing the moisture that reaches the steel substrate and that helps corrosion.
non-Uisible Contaminants
Other forms of debris, not visible to the naked eye, are chemical contaminants. The most dangerous forms of chemical contaminants are soluble salts such as chlorides and sulfates. When such contaminants are painted over, they have the power to draw the moisture through the coating to cause blistering, detachment, and accelerated corrosion of the underlying steel.
When structural steel is going to be repainted, areas that were previously rusted and pitted may contain soluble salt contamination, especially in the bases of the pits. Dry abrasive blasting typically does not remove these salts, so it is wise to check for their presence with specially designed field test kits before painting and then to take additional cleaning steps to remove the salts, if they are present in detrimental amounts. Testing for and removal of soluble salts will be discussed in detail in a later lesson.
Degrees of Surface Preparation
In any job specification, the degree of cleaning (Fig. 2 on p. 24) required for a given steel substrate before painting depends on a number of factors. The service environment of the coating system is perhaps the most important and, normal ly, is the first consideration when determining the degree of surface preparation. Generally, the more severe the environment, the better the surface preparation required. Severe service environments include immersion in liquids, exposure to aggressive chemicals or environments, high temperatures, or combinations of these conditions.
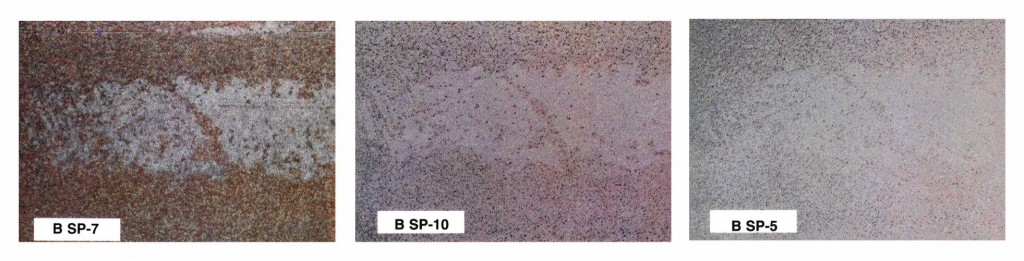
A second consideration is the generic kind of coating used. Some coatings, such as oils and alkyds, because they flow out and wet the surface well, can tolerate application over minimally prepared or hand-cleaned surfaces. In addition, some epoxy mastics and other “surface-tolerant” coatings are formulated to be applied over hand- and power tool-cleaned surfaces. Coatings such as inorganic zincs, however, are at the other end of the spectrum. They require a higher degree of cleaning than many other types. Cost is another factor in selecting the degree of surface preparation. Blast cleaning to SSPC-SP 5 (White Metal) is about 4-5 times more costly than to SSPC-SP 7 (Brush-Off) or SSPC-SP 3 (Power Tool). In some severe environments and with some coating types, rigorous cleaning is necessary; but in other instances, the cost and cost-benefit of higher grades of cleaning relative to increased coating lifetime will become an important factor in selecting the degree of surface preparation.
Finally, regulations may have an impact on the degree and method of surface preparation. In residential or are being removed, environmental and hazardous waste regulations may require containment and use of special surface preparation methods.
Determining the degree of surface preparation, as described above, is the job of a specifier or engineer. The task of doing the work is the contractor’s. No matter what degree of surface preparation is required, it must be done thoroughly. If hand-tool cleaning is required, then all of the surface area specified must be hand-tool cleaned, after it has been first cleaned by water or solvent according to SSPC-SP 1 to remove dirt, oil, or grease. If SSPC-SP 5 is specified, then conformance with the written description of SP 5 must be achieved on all designated surfaces.
In cleaning steel, it is also important to follow the proper sequence (Fig. 3). First, you must remove dirt and other debris. It is a lot easier to sweep mounds of dirt and other loose material off a surface with a broom (or by vacuuming in the case of lead-contaminated debris) than to try to remove it with surface preparation tools. The next step is removing visible oil and grease by solvent cleaning. Then you must conduct the operation of hand tool, power tool, or blast cleaning.
Step 1: Remove loose dirt and debris.
Step 2: Remove oil and grease by solvent cleaning in accordance with SSPC-SP1.
Step 3: Conduct cleaning operation (blasting, power tool cleaning, or hand tool cleaning).
Fig. 3: Observe the proper sequence when cleaning steel
If you reverse these steps, particularly with blast cleaning, the force of the blasting abrasive may drive the debris into the roughened steel surface or profile, or spread it around as is the case with grease and oil. Then it is not easy to remove, and it may interfere with coating adhesion.
In addition, it is important to achieve the surface profile required by specifications. When the profile is too rough, the coating may not cover the peaks of the profile, and the result will be pin- point rusting. When the profile is not rough enough, the coating may not anchor well to the surface, and the result will be loss of adhesion.
To make sure that a coating system will perform well as a barrier to prevent corrosion, you must roughen the steel surface for mechanical adhesion and make sure that all debris is removed so that the coating contacts the entire surface of the steel. In achieving these two conditions of cleanliness and profile, you will have assured that a proper foundation has been created for application of a coating system. This good foundation should help to provide many years of service life for the coating.
